Hippocampal Area CA2
The hippocampus is required to form new declarative memories, but the contributions of area Cornu Ammonis 2 (or CA2) to hippocampal function are virtually unknown. Recent evidence indicates that CA2 may play an important role in mediating social memory and social aggression. Pyramidal neurons in area CA2 differ significantly from those in other hippocampal subfields, especially in regards to their morphological characteristics, afferent and efferent connectivity, intrinsic and synaptic properties, and gene and protein expression profiles. But perhaps one of the most notable characteristics of area CA2 is its resistance to multiple forms of activity-dependent synaptic plasticity. The ability to induce changes in synaptic efficacy in area CA2 differs dramatically from other hippocampal subfields in that Schaffer collateral inputs originating from area CA3 fail to support the induction of activity-dependent long-term potentiation (LTP) at synapses in CA2. Curiously, CA2 pyramidal neurons possess the intracellular machinery required to support induction of activity- and NMDA receptor-dependent LTP, but the high intrinsic calcium buffering and extrusion capacity of CA2 neurons normally prevents induction from occurring. Even more interesting is the observation that Schaffer collateral projections to CA2 are, indeed, quite plastic, but only in response to specific neuromodulatory signals (such as, adenosine or vasopressin). Multiple complimentary techniques are used in PlasticityLab to extend our limited knowledge and understanding of how such neuromodulatory signals can influence this relatively uncharacterised component of the hippocampal circuit.
Hippocampal area CA2 is often excluded from circuit diagrams illustrating the flow of information through the hippocampus. The simplified schematic diagrams (A & B) highlight how area CA2 (shown in red) fits within the traditional “trisynaptic” circuit. (B) Schaffer collateral projections from area CA3 to CA2 synapse in the stratum radiatum at a location that is proximal to the cell body layer, whereas temporoammonic inputs from the entorhinal cortex to CA2 synapse distally in the stratum lacunosum moleculare. There is evidence to suggest that the induction of activity-dependent LTP differs significantly in these two independent inputs to the same CA2 neurons.
Area CA2 is a distinct component of the hippocampal circuit. It is possible to isolate CA2 from other hippocampal subfields by targeting genes that are highly expressed in CA2 pyramidal neurons.
The lack of activity-dependent LTP at Schaffer collateral synapses in area CA2 is due mainly to robust calcium handling in CA2 pyramidal neurons. (A,B) Pairing 3-Hz afferent synaptic stimulation of the Schaffer collaterals with the depolarization of CA2 pyramidal cells to 0 mV fails to induce activity-dependent LTP in area CA2. Group data in B show the averaged amplitudes of EPSCs (normalized to baseline) before and after the pairing protocol (arrow) to induce LTP in CA2 neurons. Bars indicate the mean + SEM in this and subsequent figures. Sample EPSCs shown in A are from the time points marked by the corresponding numbers in B. (C,D) In contrast, the same pairing protocol induces robust LTP in Schaffer collateral inputs to area CA1. The intrinsic biophysical properties of neurons in CA2 differ significantly from those in CA1. Relative to pyramidal cells in area CA1, CA2 pyramidal neurons show greater leak currents at holding potentials less than 60 mV (E, red circles, arrow), as well as have a lower resting membrane potential (F, red bar), higher rheobase current (G, red bar), and lower action potential threshold (H, red bar). Note, stars indicate P < 0.05. Data modified from Zhao et al. (2007). Differences in intrinsic excitability alone, however, do not account for the lack of LTP in CA2 neurons (data not shown). CA2 pyramidal neurons display higher calcium buffering and extrusion relative to cells in CA1. (I) A temporary increase in the amount of extracellular calcium from 2 mM (gray circles) to 10 mM (red circles) for 3 min (indicated by the red bar) permits induction of LTP in CA2 pyramidal cells following tetanic stimulation (200 Hz HFS; arrow) of the Schaffer collaterals. (J) Loading CA1 neurons with a functional analog of the calmodulin-regulating protein Pep-19 blocks induction of LTP (blue circles). Data modified from Simons et al. (2009).
Caffeine and Hippocampal Area CA2
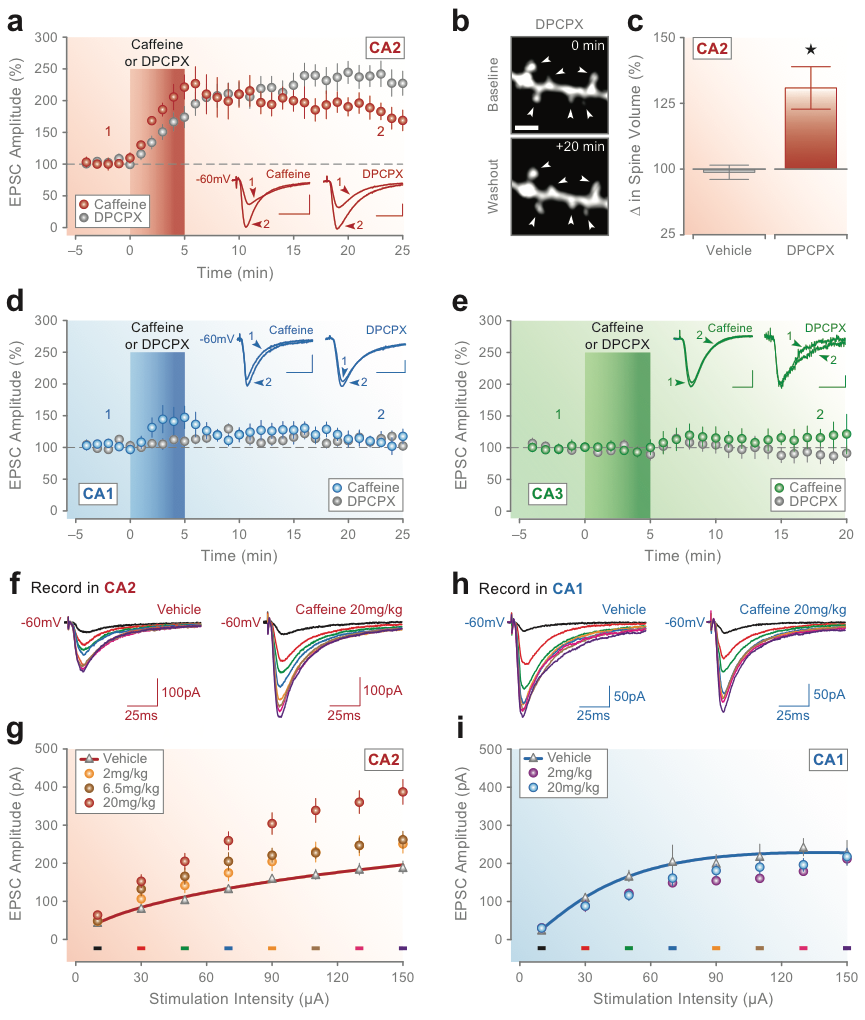
Adenosine acts as a neuromodulator in the brain, and signalling at adenosine receptors in the hippocampus has been shown to influence memory formation and behaviour by regulating synaptic plasticity. Caffeine is a naturally-occurring cognitive enhancer that is widely-consumed to improve attention and augment memory. Its primary mechanism of action is thought to be through blockade of adenosine A1 receptors (A1Rs). Interestingly, A1Rs are highly expressed in pyramidal neurons in CA2 when compared to the rest of the hippocampal formation, and caffeine selectively enhances excitatory synaptic transmission in CA2 at concentrations that have little effect on responses evoked in other hippocampal subfields. Specifically, application of caffeine to hippocampal slices facilitates synaptic responses in Schaffer collateral inputs to area CA2, but not in the same inputs to CA1, nor in mossy fibre projections from the dentate gyrus to area CA3. This enhanced sensitivity to caffeine is related not only to the high expression of A1Rs in CA2, but also to an array of additional downstream signalling enzymes that are also highly enriched in CA2 neurons. In addition, two-photon confocal imaging of live CA2 pyramidal neurons in vitro has also shown that the A1R-mediated increase in synaptic efficacy is accompanied by a coincident change in the volume of spines located along branches of apical dendrites. Given the importance of experience-driven modifications in synaptic function to learning, memory and cognition, A1Rs in CA2 may play a significant role in mediating the cognitive-enhancing effects of caffeine. Understanding how caffeine and other neuromodulatory signals influence synaptic function in CA2 to modulate cognition and behaviour is a major focus of research in PlasticityLab.
Caffeine affects synaptic physiology in hippocampal area CA2.
The adenosine A1 receptor is highly expressed in CA2 pyramidal neurons relative to the rest of the hippocampus (image modified from Ochiishi et al., 1999).
Blockade of A1 receptors potentiates synaptic responses in CA2 but has no lasting effect on synaptic transmission in areas CA1 or CA3. (A) Bath-application of caffeine (100 mM) or the selective A1R antagonist DPCPX (10 nM) for 5 min potentiates synaptic responses in CA2. The red bar in A marks the onset and duration of caffeine or DPCPX application, and the inset traces show example currents recorded at the latencies marked by the corresponding numbers. Brief exposure to DPCPX increases the volume of spines located on apical dendritic branches of CA2 pyramidal neurons. (B) Two-photon confocal images of a spine-containing segment of secondary apical dendrite for a CA2 neuron loaded with Alexa Fluor 594 are shown before (0 min; baseline) and after (+20 min; washout) 5-min application of DPCPX (10 nM). Arrows in B mark spines that showed a significant change in volume after treatment with DPCPX (calibration bar ¼ 1 mm). (C) Group data for all spines are shown comparing the average change in spine volume at 20-min post-vehicle or post-DPCPX treatment. There was no change in the amplitude of synaptic responses induced by caffeine or DPCPX (D) in areas CA1 or CA3 (E). Conventions in D and E are the same as in A (calibration bars for inset traces in A, D, and E: 50 pA, 25 msec). Caffeine consumption in vivo induces synaptic potentiation in hippocampal CA2 but not in CA1. (F, G) Oral administration of caffeine induces a dose-dependent increase in the amplitude of synaptic responses in CA2 in vitro across a range of stimulation intensities. (H, I) Treatment with caffeine, however, had no effect on responses in CA1. Group data in G and I show average synaptic responses at each stimulation intensity and dose of caffeine tested. Colored bars in G and I indicate the intensity used to evoke the corresponding colored currents in F and H from single neurons in slices from rats dosed with 20 mg/kg caffeine or vehicle (CA2, F; CA1, H). Data modified from Simons, Caruana et al. (2011).